NPM1 Fragment Analysis Panel, NPM1 FRG 1.1
NPM1 Fragment Analysis Panel and NPM1 FRG 1.1 Kit: A Powerful Diagnostic Tool for Acute Myeloid Leukemia (AML)
In a significant portion of patients with cytogenetically normal acute myeloid leukemia (AML) (approximately 60%), mutations in exon 12 of the NPM1 gene are detected. Individuals with these mutations are identified as NPM1 positive, and there is a negative correlation with CD34 expression. Studies have revealed the presence of around 40 mutations, often manifesting as 4-base insertions.

FEATURES
- Analysis of NPM1 mutation status
- Identification of mutation type (A, B, or D)
- Optimal quality control and ISO 13485 certification
- Easy workflow with ready-to-use solutions
DATA INTERPRETATION
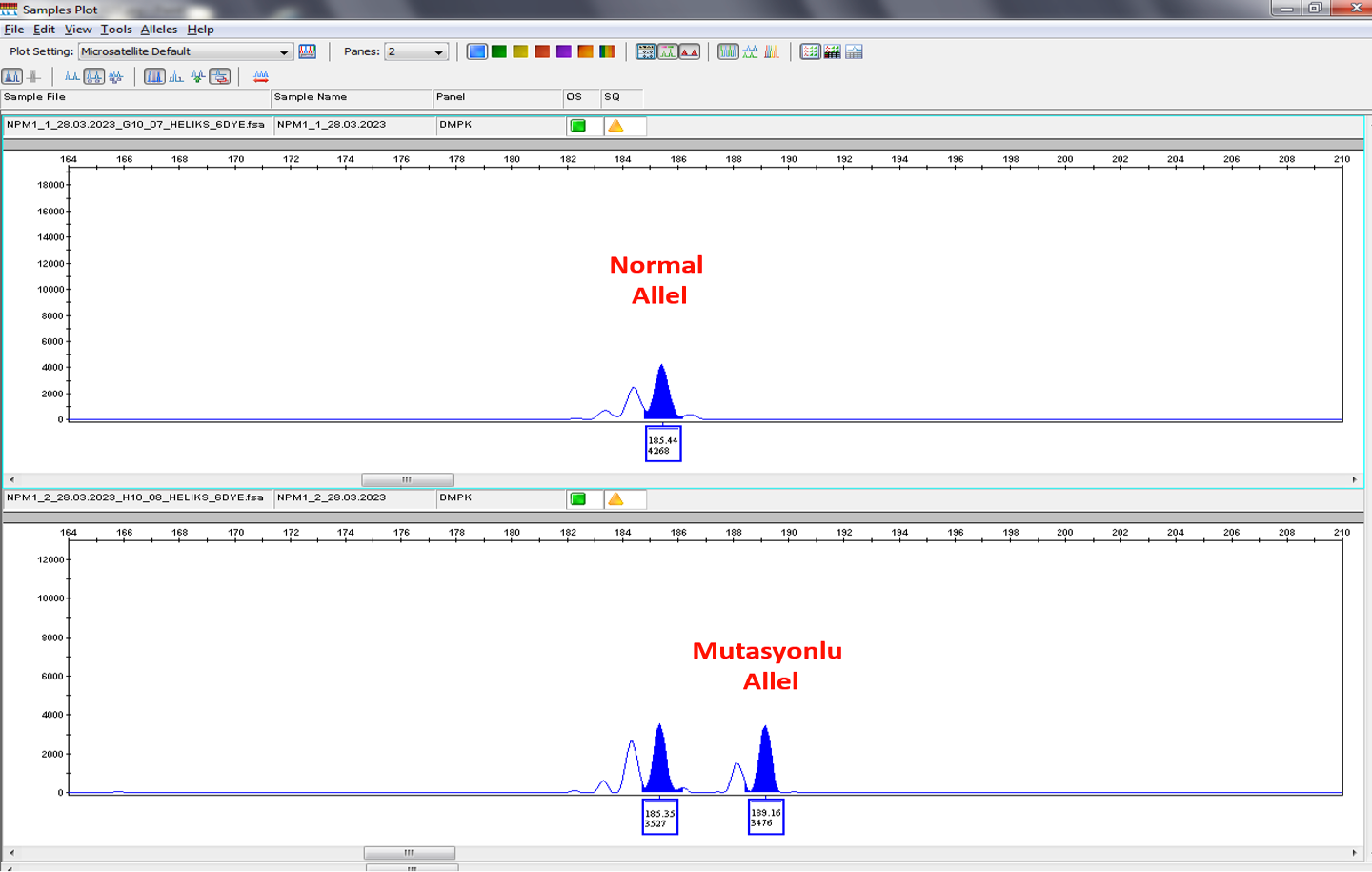
Figure 1: GeneMapper image of individuals positive and negative for NPM1 gene mutations
ORDER INFORMATION
|
CATALOG NO |
03001025: NPM1 Fragment Analysis Panel, NPM1 FRG 1.1 (25 Tests) |
|
03001050: NPM1 Fragment Analysis Panel, NPM1 FRG 1.1 (50 Tests) |
|
|
03001100: NPM1 Fragment Analysis Panel, NPM1 FRG 1.1 (100 Tests) |
|
|
SAMPLE TYPE |
Whole Blood in EDTA Tube |
|
TEST CONTENT |
dH2O, PCR Mix, DMSO, NPM1 Primer Mix, Taq Pol. |
|
TARGET GENES and POINTS |
NPM1 insertions |
|
STORAGE CONDITIONS and SHELF LIFE |
Should be stored at -20°C in the dark. Shelf life is 12 months. |
|
COMPATIBLE DEVICES |
All PCR Devices, ABI 3130, 3500, and other genetic analyzers |
